Changing the paradigm of diagnosis for Chronic Liver Diseases
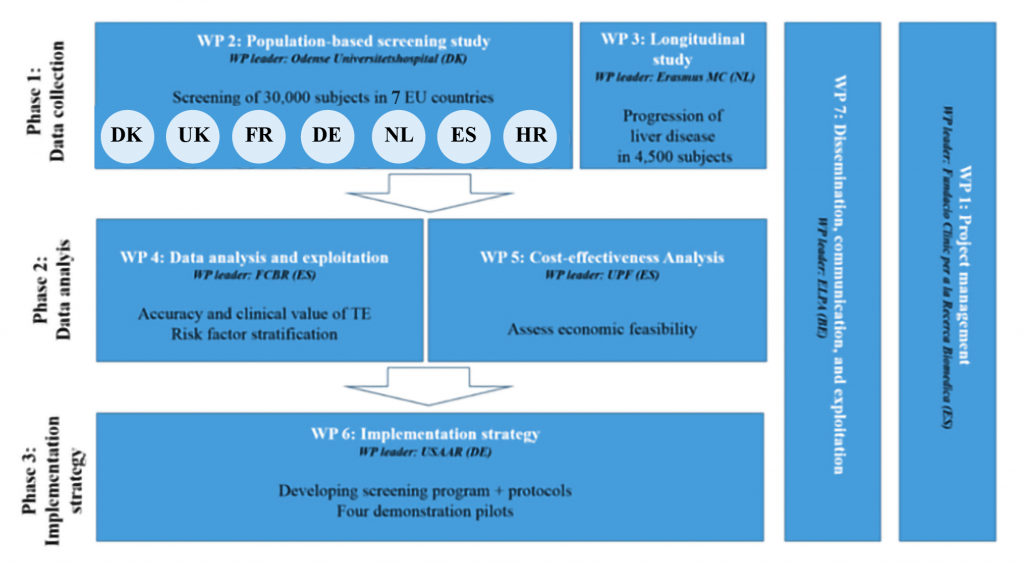
The goal of this work package is to guarantee coordination, motivation and effective interaction among the LiverScreen partners leading to successful progression of the project within agreed timelines, costs and quality described by the project.
The objective of this WP is to perform a cross-sectional study to generate data from the general population regarding prevalence of liver fibrosis and prevalence of risk factors associated to chronic liver diseases. The screening study will be conducted in 14 medical centres across 8 European countries, where we will perform screening for chronic liver disease in 30,000 randomly selected subjects from the general population, aged 40-75 years. This data will serve as input for WP4 and WP5.
The objective of this WP is to perform a longitudinal study in 3 pre-existing cohorts from 3 Consortium partners (Fundació Clínic per a la Recerca Biomèdica, Erasmus Universitair Medisch Centrum Rotterdam, Odense Universitetshospital) that were previously assessed with Transient Elastography (TE) for liver fibrosis detection, will be followed-up and re-assessed with TE after a minimum of 4 years from the first assessment. The 3 previous study cohorts include a total of 4,500 subjects, 2,100 from Barcelona (Clin Gastroenterol Hepatol. 2018 Jul;16(7):1138-1145.e5), 1,400 from Rotterdam (Koehler EM et al., Hepatology 2016;63:138-147) and 1,000 from Odense (not published). This WP will generate date regarding liver fibrosis progression (prevalence and risk-factors). These cohorts will be tested on different parameters indicating liver disease. This data will serve as input for WP4 and WP5.
This work package will provide a comprehensive analysis and exploitation of the data gathered in WP2 and WP3. This entails:
1) Comparison of the TE results with the corresponding liver biopsies and known serum biomarkers for liver fibrosis generated in WP2, for accuracy and validation of TE technology as a diagnostic tool in the general population;
2) Analyses of all characteristics of the participants generated in WP2 and WP3 to identify risk factors (both known and new) associated with the prevalence of liver fibrosis;
3) Apply machine learning techniques on the longitudinal data of WP3 to develop a prognostic model of liver fibrosis and related health outcomes;
4) Combine both the pre-test risk factors and the post-test prognosis into a digital tool application for health care providers and patients.
Data generated in WP2, WP3 and WP6 are combined to estimate the impact of the LiverScreen program on benefits and harms on the screening population and to estimate the health and economic consequences of the LiverScreen program. In terms of efficiency of LiverScreen, we will perform a cost- effectiveness analysis to estimate the incremental cost-effectiveness ratios of liver fibrosis preventive screening adoption for different populations and European public healthcare systems. In terms of sustainability, a budget impact analysis will be carried out. In terms of context-specific implementation, we will estimate the optimal screening adoption and location given the geographic, socio-demographic and healthcare contexts in the European Union.
Data from all previous work packages will be used in this work package to develop a specific screening program for large-scale adoption across Europe. We have identified 4 pilot regions, which are strategically chosen to represent the wide range of health care and socio-economic differences in Europe. These pilot regions are Saarland (Germany), Zagreb region (Croatia), Catalonia (Spain), and Southern Denmark (Denmark). For all regions, governmental letters of support are obtained and attached in the annex of the proposal. The screening program has been pre-defined following 7 steps, but the implementation strategy will be tailored according to particularities and risk factors prevalence of each of 4 regions:
The results from these tailored implementation pilots will form the basis for the development of a more general implementation strategy. This general implementation strategy can be used by government institutions and health care organisations across Europe to provide a framework for the implementation of a screening program for chronic liver disease (CLD) in all European countries (generalization of LiverScreen) and possibly beyond.
The objectives of this WP are: